A trending concept that we've been doing for decades.
Leveraging our pioneering expertise in rare diseases, our solutions aim to connect investigators to patients in the most pragmatic ways, using technology to remove barriers to enrolment and retention.
Decentralized and virtual trials are a trending concept, yet Ergomed has been doing them for decades.
As pioneers in orphan drug research, we have been implementing technologies and solutions to reach hard-to-find patients in the most remote locations. Where every p-value counts, there can be no barrier to enrolment and retention, so we have developed strategies to connect patients with investigators in the most pragmatic ways.
These approaches are now needed outside rare disease studies. With the increase in technologies and the proliferation of specialist service providers, decentralized clinical trials can drive efficiency and reduce costs, especially for larger clinical studies.
Applied technologies – such as wearables, artificial intelligence (AI), cloud computing, and the deployment of 5G – can make the trial process more effective, adaptable, and convenient. The key is to introduce greater flexibility and efficiency while ensuring high-quality data and enhanced safety for patients. Our approach also places patients at the center of the research.
We use virtual solutions to improve the comfort and confidentiality of patients, as well as increasing patient recruitment and retention, and improving patient diversity. What’s more, virtual solutions can improve data collection and reduce clinical trial costs.
Technology is important but success with decentralized trials hinges on the clarity of intent when developing the protocol to be executed in a decentralized fashion. There is no substitute for the early engagement of highly skilled, multidisciplinary, and experienced specialists to design these technically and operationally challenging studies.
Early engagement is essential for appropriate endorsement and approval, especially as it can take time to become accustomed to virtual interactions. Each protocol needs to be reviewed and fully discussed on a case-by-case basis.
The world of virtual technologies, tools, and services is endless, so we have assessed vendors and evaluated their products and services in order to advise on which solutions are most appropriate for a specific trial.
The Ergomed team can also advise and perform hybrid clinical trials, which combine virtual and traditional methods.
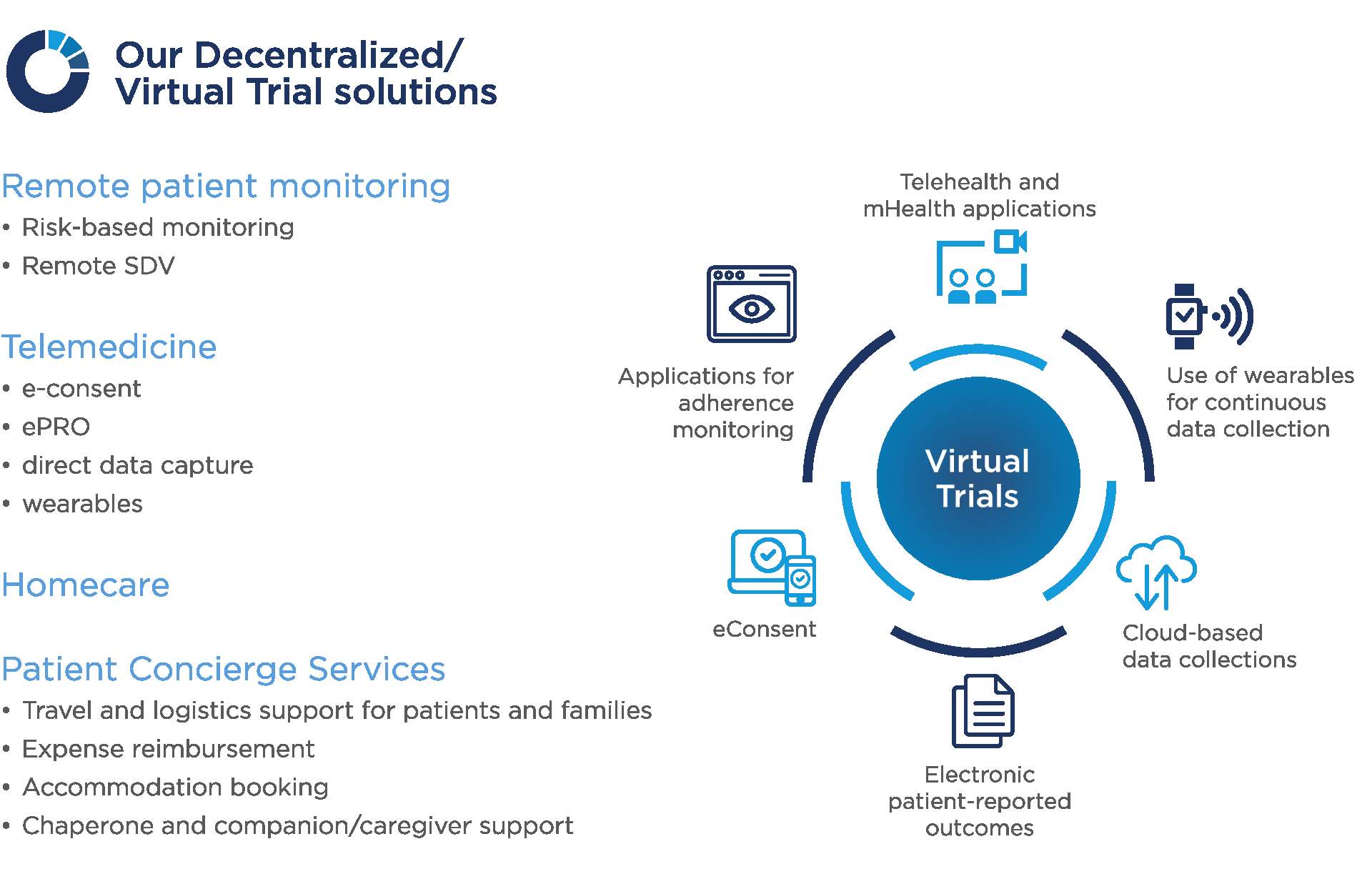
Specific virtual solutions include:
Risk-based monitoring
With risk-based monitoring strategies, the volume and frequency of monitoring are commonly reduced, as data is only verified at high-risk sites based on triggered events or certain pre-defined critical events in the study. The quality of the trial data can be improved by identifying, assessing, monitoring, and mitigating risks at the earliest opportunity. We combine platforms and extensive medical and research knowledge to identify key indicators of potential issues in trial conduct, reducing the burden on the study team and allowing them to be laser-focused on the key factors in the project.
Remote SDV
Through our remote model, we verify data with reduced visits to sites. Personalized to your needs, combining traditional and alternative approaches, while ensuring the data collected are of high quality and integrity.
Telemedicine approaches (e-consent, ePRO, direct data capture models, etc.) allow for the evaluation and treatment of patients remotely through technology. This approach has evolved over the last decades and is becoming an increasingly important part of clinical trials, especially where the patient burden is a factor (e.g. reducing travel time to clinics, etc.).
Wearable Devices
We are moving rapidly towards remote monitoring and real-time detection of illnesses mediated by a range of wearable devices that impose fewer limits on patients’ daily lives and offer better control of medical conditions.
Homecare
Administering treatments and monitoring outcomes in everyday or home settings offer a range of advantages to both patient and sponsor. They include reduced family and patient burden, reduced time, costs, and effort by reducing the need or number of visit sites and for making changes to everyday activities. A reduced burden for patients leads to higher recruitment and retention rates.
However, implementation of successful homecare solutions requires a deep understanding of local regulatory requirements, as well as cultural, legal, and insurance implications. Our global network of clinical operations and site management leads are on hand to advise on the suitability and practicality of employing homecare in clinical trials.
Patient Concierge
Participants in clinical trials frequently need specialized support; obstacles can include travel to a distant study site, scheduling multiple appointments, etc. Our patient concierge services offer personalized assistance to patients and their families around trial comprehension, logistics, technology, and non-medical issues and questions, enhancing retention.